Health monitors and medical devices are more popular than ever. But what if these machines caused more harm than good? This is where the FDA comes in. Through FDA registration and approval, medical devices are safety tested before they can be marketed and sold in the U.S. These stringent protocols help protect consumer health and keep ineffective products off the market.
However, there are many different types of FDA statuses like: FDA Registered vs FDA Approved vs FDA Cleared.
What’s the difference between these statuses and how are devices tested to ensure safety? This guide breaks it down for you.
Importance of FDA Approval for Medical Devices
The Center for Devices and Radiological Health (CDRH), a body within the FDA, is responsible for demonstrating the safety and effectiveness of all medical devices.
The medical industry seeks approval from the FDA to ensure everything is done in compliance with the law. With FDA approved medical devices, manufacturers must prove that:
# They have tested devices for efficiency and safety
# Manufacturing practices and quality control standards have been met
# They have adhered to the guidelines for labeling, promotion, etc.
What is FDA Approval?
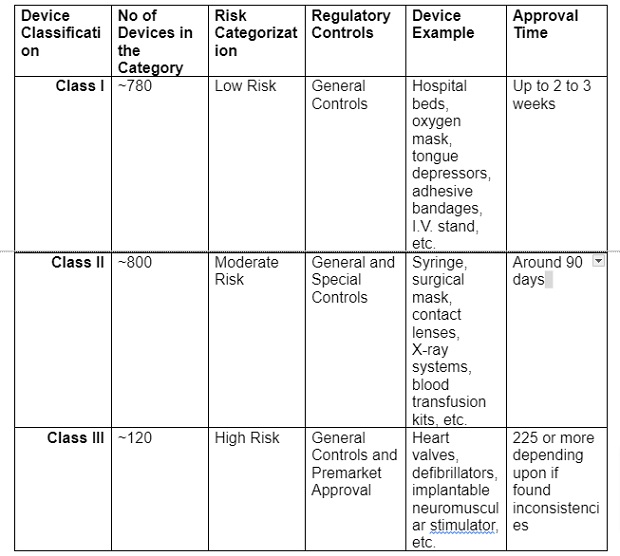
The FDA uses a risk-based, tiered approach for regulating medical devices for people. Medical devices are classified into three categories: Class I, II, and III.
Device classification is based on the level of regulatory controls needed and the risk associated with a device. An implanted heart monitor, for example, must go through stricter protocols than an adhesive bandage.
Ultimately, each classification is associated with different regulatory requirements.
In fact, the FDA has classified about 1700 generic groups of devices. These devices are classified within 16 medical specialties.
The table below gives you a brief overview of the device classification and how long FDA approval can take for a device in each class:
Classification System and Risk Categorization:
A Closer Look at FDA Medical Device Classes
– Class I general controls are:
– Establishment registration and listing
– Adulteration/Misbranding
– Labeling
– Quality system regulation
– Premarket notification [510(k)], unless exempt
– Medical Device Reporting (MDR)
Class II (above listed general controls plus) special controls are:
– Mandatory performance standard
– Special labeling
– Guidelines
– Recommendations or other actions
– Surveillance after product launch
Class III (general and special controls aren’t sufficient) requires:
– Premarket approval (PMA) submission
– [510(k)] is required instead of PMA (in some cases)
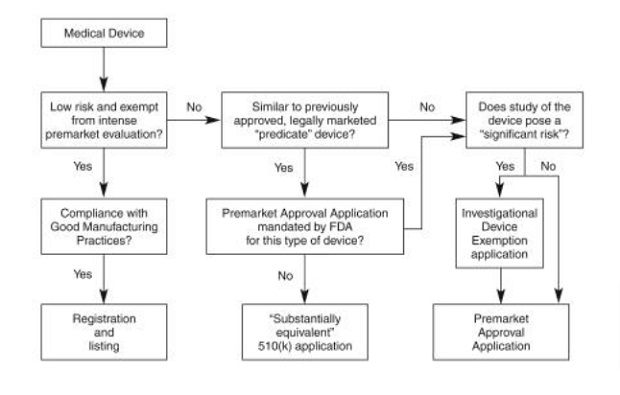
Below is a schematic representation of the medical device approval process.
How to Get FDA Approval?
FDA approval for the medical devices process can briefly be stated as below:
1. Certification of the device as per the Class I, II, or III classification
2. Creating a prototype and testing its efficacy for the intended use
3. Pathway to approval
a) Premarket notification [510(k)]
b) Premarket approval (PMA)
c) Humanitarian Device Exemption (HDE) is used to treat diseases or conditions that affect a small population
4. Device review and approval
5. FDA post-market device performance monitoring
Ensuring Safety and Efficacy
The Food and Drug Administration protects public health by regulating the safety, efficacy, and security of drugs, vaccines, biological products, medical devices, tobacco, cosmetics, and products that emit radiation.
FDA also promotes innovation that makes medical products more effective, safer, and affordable.
It also helps the public to get accurate, scientific information they need to use medical products for effective diagnosis and improve health or treatment for life-threatening conditions.
Gaining Market Access
The FDA created various labels to oversee medical device processes, such as FDA Registered, FDA Approved, FDA Cleared, etc.
Companies have often misunderstood and opted for these terms for marketing purposes.
Let’s understand the difference between FDA Registered vs FDA Approved vs FDA Cleared:
FDA Registered
It signifies that the business has fulfilled its yearly regulatory obligation by paying an ‘Annual Establishment Registration Fee’ and filing with the FDA.
FDA Approved
This status means that the FDA experts and agency have concluded that the ‘benefits of the product outweigh the known risks for the intended use.’ Manufacturers must submit a premarket approval (PMA) application and the clinical testing results.
Not all products undergo premarket approval, that is, an approval status, before a product can be sold to consumers.
FDA Cleared
This indicates that the FDA permits a manufacturer to market the device through the 510(k) process. Which means that a similar or substantial equivalent product is already marketed that has FDA clearance or approval.
Those already cleared products are called a predicate, and you can legally market them.
Building Trust and Confidence
FDA approval and guidelines dictate that the medical industry ensures the delivery of fair and satisfactory healthcare products.
FDA approved medical devices build trust and credibility among patients, healthcare providers, and regulatory agencies.
However, to receive accreditation and approvals, businesses must ensure the following:
# Safety and Efficacy
# Preclinical testing
# Clinical trials
# Demonstrate compliance with regulations
# Improving quality and efficiency
# Enhancing transparency and accountability
Compliance and Enforcement
Whether designing medical devices or working in the quality system, regulations make a massive difference as they ensure that clinicians and patients are utilizing medical technology that is both safe and effective.
The quality system regulation – 21 CFR Part 820
For medical device manufacturers, the quality system regulation includes requirements related to the methods used and the facilities and controls used for:
# Designing
# Purchasing
# Good Manufacturing Practices
# Packaging
# Labeling
# Storing
# Installing and servicing medical devices.
Manufacturing facilities must sometimes undergo FDA inspections to ensure compliance with the QS requirements.
Challenges and Considerations
FDA approval for medical devices can be tedious sometimes. Below mentioned are a few common challenges faced by companies during the medical device approval process:
# Cross-functional understanding of regulations such as FDA, EMA, and CFDA
# Risk management includes technical and patient safety concerns, legal liabilities, etc.
# Data security for HIPAA compliance
# Clinical evaluation and evidence
# Regular inspections
# Distributors owning the regulatory approvals
# Complying with unique country-specific approvals
# Fund management
# Lack of workforce
Wrapping Up
FDA approved medical devices bring a sense of safety and security among the medical industry community. Though the medical device approval process seems lengthy and tedious, the FDA must abide to protect public health with enough regulation and compliance in place.
It is in the best interest of the public and healthcare providers to ensure that they choose an FDA approved medical device for treating their medical condition. However, avoid falling prey to marketing tactics if the device says FDA Registered, FDA Approval, or even sometimes FDA Granted.